1 Methylcyclohexene To 2 Methylcyclohexanone
salachar
Sep 13, 2025 · 5 min read
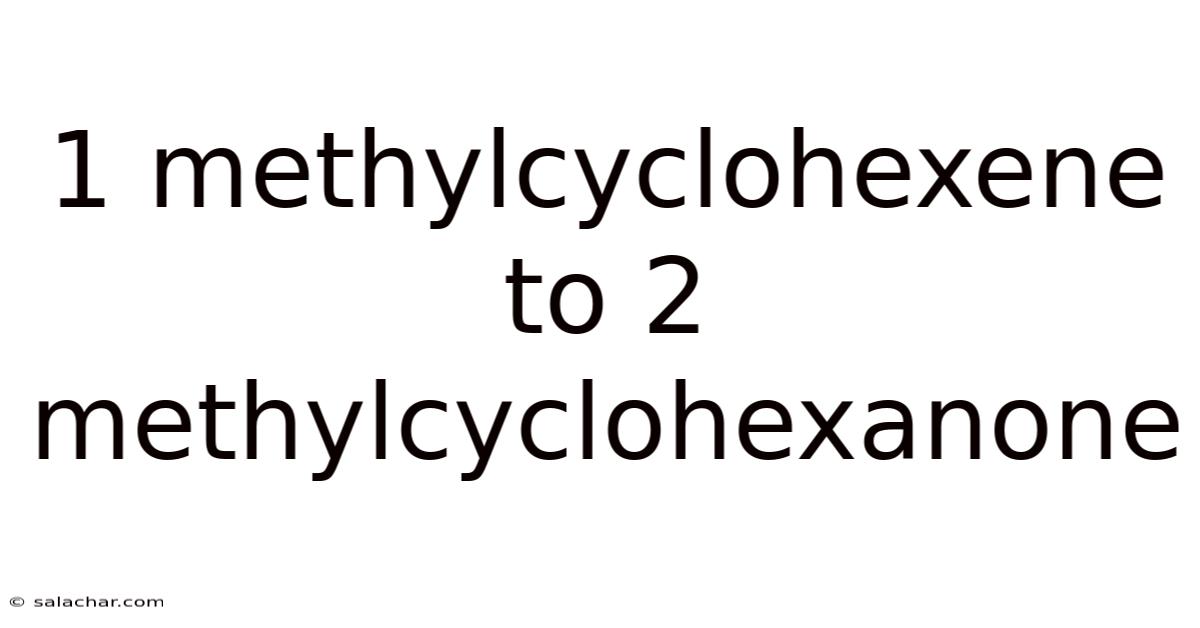
Table of Contents
The Oxidation of 1-Methylcyclohexene to 2-Methylcyclohexanone: A Comprehensive Guide
The transformation of 1-methylcyclohexene to 2-methylcyclohexanone represents a classic example of organic oxidation, specifically the oxidation of an alkene to a ketone. This reaction is crucial in organic synthesis, providing a versatile route to synthesize valuable ketone compounds. Understanding the mechanism, reaction conditions, and potential side reactions is essential for successful execution and optimization of this conversion. This comprehensive guide will delve into the intricacies of this transformation, providing a detailed explanation accessible to both beginners and experienced chemists.
Introduction: Understanding the Reaction
The conversion of 1-methylcyclohexene to 2-methylcyclohexanone involves the addition of an oxygen atom to the carbon-carbon double bond of the alkene. This process, known as oxidation, can be achieved through various reagents and reaction conditions. The key to achieving a high yield of 2-methylcyclohexanone lies in controlling the reaction's selectivity, minimizing the formation of unwanted byproducts. The desired product, 2-methylcyclohexanone, is a six-membered cyclic ketone with a methyl group at the 2-position. This specific arrangement of atoms is crucial for its application in various chemical syntheses.
Reaction Mechanism: Unveiling the Steps
Several oxidizing agents can facilitate the conversion of 1-methylcyclohexene to 2-methylcyclohexanone. The most common pathways involve the formation of an epoxide intermediate followed by ring opening and rearrangement. Let's examine a common mechanism using a peroxyacid like meta-chloroperoxybenzoic acid (mCPBA):
-
Epoxide Formation: The reaction initiates with the electrophilic attack of the peroxyacid on the alkene's double bond. This attack results in the formation of a three-membered ring called an epoxide. The oxygen atom from the peroxyacid is incorporated into the ring. This step is typically stereospecific, meaning the stereochemistry of the starting alkene is retained in the epoxide.
-
Acid-Catalyzed Ring Opening: The epoxide is relatively unstable and undergoes ring-opening in the presence of an acid catalyst. The acid protonates the epoxide oxygen, making it a better leaving group. Nucleophilic attack by water (or another suitable nucleophile) opens the ring, resulting in the formation of a vicinal diol (a molecule with two hydroxyl groups on adjacent carbons).
-
Oxidation to Ketone: The vicinal diol undergoes further oxidation, typically using an oxidizing agent like chromic acid or Jones reagent (chromic acid in acetone). This step converts the two hydroxyl groups into a carbonyl group (C=O), resulting in the formation of 2-methylcyclohexanone. This oxidation step involves the removal of two hydrogen atoms from the vicinal diol, ultimately forming the ketone.
Alternative Oxidation Methods: Exploring Different Pathways
While the mCPBA/acid-catalyzed pathway is common, other oxidizing agents can also achieve the desired transformation. These include:
-
Ozone (O3) followed by reductive workup: Ozonolysis cleaves the double bond, forming an ozonide intermediate. A reductive workup, such as with zinc and acetic acid, converts the ozonide into a carbonyl compound. While effective, this method may require careful control of reaction conditions to prevent over-oxidation.
-
Potassium permanganate (KMnO4): This strong oxidizing agent can directly convert the alkene to the ketone, although it often requires specific reaction conditions to avoid over-oxidation to carboxylic acids. The use of KMnO4 often leads to a mixture of products.
-
Osmium tetroxide (OsO4): This reagent forms a cyclic osmate ester, which can be further hydrolyzed to a vicinal diol. Subsequent oxidation of the diol yields the ketone. However, OsO4 is toxic and expensive, limiting its use in large-scale syntheses.
Optimizing the Reaction: Factors Affecting Yield and Selectivity
Several factors can significantly influence the yield and selectivity of the 1-methylcyclohexene to 2-methylcyclohexanone conversion:
-
Choice of Oxidizing Agent: The selection of the appropriate oxidizing agent is paramount. mCPBA offers a good balance between reactivity and selectivity, while other reagents may require more precise control of reaction conditions.
-
Reaction Temperature and Time: The reaction temperature and time must be optimized to ensure complete conversion of the alkene without the formation of unwanted byproducts. Too high a temperature or prolonged reaction times can lead to over-oxidation.
-
Solvent Selection: The choice of solvent can influence the reaction rate and selectivity. Appropriate solvents must be inert to the reactants and reagents.
-
Acid Catalyst (if applicable): The type and concentration of the acid catalyst (in the case of the epoxide pathway) can impact the efficiency of the ring-opening step.
-
Purification Techniques: Efficient purification methods, such as distillation or chromatography, are essential to isolate the desired 2-methylcyclohexanone from any remaining starting material or byproducts.
Practical Considerations and Safety Precautions
Working with oxidizing agents requires careful attention to safety precautions:
-
Protective Equipment: Always wear appropriate personal protective equipment (PPE), including gloves, safety goggles, and a lab coat.
-
Ventilation: Ensure adequate ventilation in the laboratory, as many oxidizing agents can produce irritating or toxic fumes.
-
Proper Handling: Handle oxidizing agents carefully and avoid contact with skin or eyes.
-
Waste Disposal: Dispose of chemical waste according to the appropriate guidelines and regulations.
Frequently Asked Questions (FAQ)
Q1: What are the potential side reactions in this conversion?
A1: Potential side reactions include the formation of epoxides that don't readily open, over-oxidation to carboxylic acids, and the formation of other isomeric ketones.
Q2: How can I determine the purity of the obtained 2-methylcyclohexanone?
A2: Purity can be determined using various techniques, including gas chromatography (GC), high-performance liquid chromatography (HPLC), and nuclear magnetic resonance (NMR) spectroscopy.
Q3: Can this reaction be scaled up for industrial applications?
A3: Yes, this reaction is readily scalable, although the choice of oxidizing agent and reaction conditions might need optimization for large-scale production.
Q4: What are some common applications of 2-methylcyclohexanone?
A4: 2-Methylcyclohexanone is a valuable intermediate in the synthesis of various pharmaceuticals, fragrances, and other fine chemicals.
Conclusion: A Versatile and Essential Transformation
The oxidation of 1-methylcyclohexene to 2-methylcyclohexanone is a fundamental transformation in organic chemistry, offering a straightforward route to synthesize a valuable ketone compound. Understanding the reaction mechanism, optimizing reaction conditions, and adhering to safety protocols are crucial for achieving high yields and selectivity. The versatility of this reaction and the importance of 2-methylcyclohexanone in various applications underscore its significance in both academic research and industrial processes. This detailed guide provides a solid foundation for anyone seeking to understand and execute this essential organic reaction. Further investigation into specific oxidizing agents and reaction optimization techniques will lead to a deeper understanding of this valuable transformation. Remember to always prioritize safety when conducting chemical experiments.
Latest Posts
Latest Posts
-
How Does Amoeba Get Food
Sep 13, 2025
-
Characteristics Of A Pure Substance
Sep 13, 2025
-
What Does R C C Stand For
Sep 13, 2025
-
Labelled Diagram Of A Planaria
Sep 13, 2025
-
150 Kg How Many Pounds
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about 1 Methylcyclohexene To 2 Methylcyclohexanone . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.