Ca Oh 2 Molar Mass
salachar
Sep 12, 2025 · 6 min read
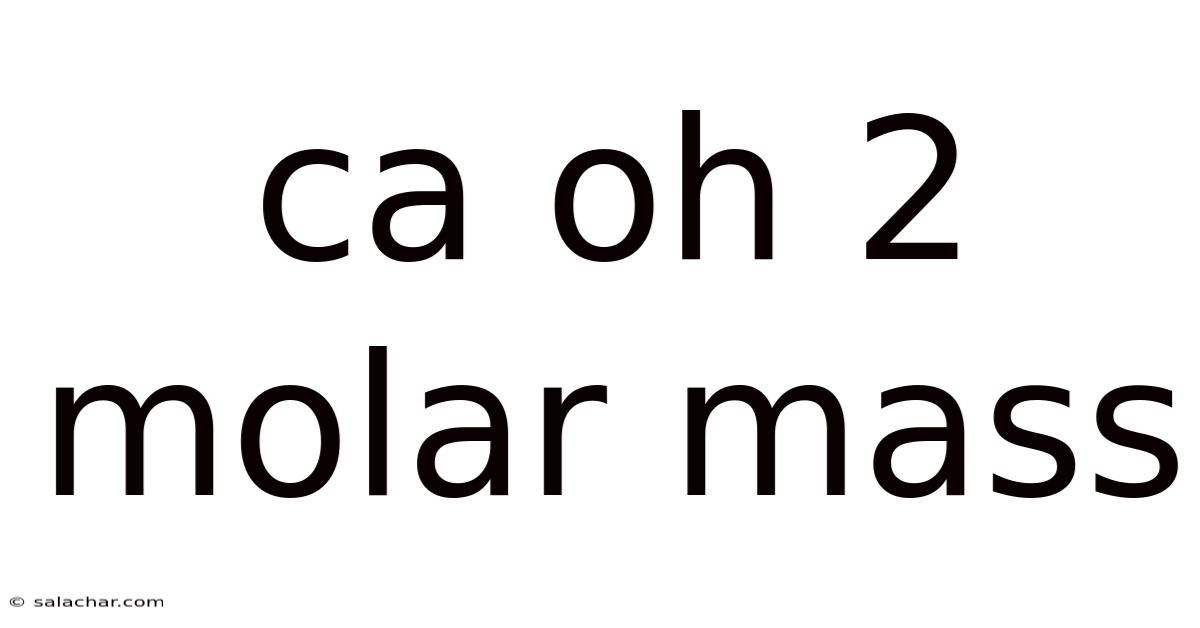
Table of Contents
Understanding Calcium Hydroxide (Ca(OH)₂): Molar Mass and Beyond
Calcium hydroxide, also known as slaked lime or hydrated lime, is a chemical compound with the formula Ca(OH)₂. Understanding its molar mass is crucial in various applications, from chemistry experiments to industrial processes. This comprehensive guide will delve into calculating the molar mass of Ca(OH)₂, exploring its properties, uses, and safety considerations. We will go beyond a simple calculation, providing a deeper understanding of this important chemical compound.
Calculating the Molar Mass of Ca(OH)₂
The molar mass of a compound represents the mass of one mole of that substance. To calculate the molar mass of Ca(OH)₂, we need to consider the atomic masses of its constituent elements: calcium (Ca), oxygen (O), and hydrogen (H). These atomic masses are typically found on the periodic table.
- Calcium (Ca): Approximately 40.08 g/mol
- Oxygen (O): Approximately 16.00 g/mol
- Hydrogen (H): Approximately 1.01 g/mol
The formula Ca(OH)₂ indicates that one molecule of calcium hydroxide contains one calcium atom, two oxygen atoms, and two hydrogen atoms. Therefore, the molar mass is calculated as follows:
Molar Mass (Ca(OH)₂) = (1 × Atomic Mass of Ca) + (2 × Atomic Mass of O) + (2 × Atomic Mass of H)
Molar Mass (Ca(OH)₂) = (1 × 40.08 g/mol) + (2 × 16.00 g/mol) + (2 × 1.01 g/mol)
Molar Mass (Ca(OH)₂) = 40.08 g/mol + 32.00 g/mol + 2.02 g/mol
Molar Mass (Ca(OH)₂) ≈ 74.10 g/mol
Therefore, the molar mass of calcium hydroxide is approximately 74.10 grams per mole. This value is essential for stoichiometric calculations, determining the amount of reactant needed in a chemical reaction, or calculating the concentration of a solution.
Properties of Calcium Hydroxide
Calcium hydroxide is a white, crystalline powder that is slightly soluble in water. Its solubility increases with decreasing temperature. This solution, known as limewater, is slightly alkaline, with a pH greater than 7. Some key properties include:
- Appearance: White powder
- Solubility: Slightly soluble in water
- pH: Alkaline (basic)
- Melting point: 580 °C (853 K; 1076 °F) (decomposes)
- Density: 2.211 g/cm³
Uses of Calcium Hydroxide
The versatility of calcium hydroxide makes it a widely used compound in various industries and applications. These include:
-
Construction and Building Materials: Calcium hydroxide is a key ingredient in mortar, plaster, and concrete. Its alkaline nature helps to bind the other components together, creating a strong and durable material. It also contributes to the setting and hardening process.
-
Wastewater Treatment: In wastewater treatment plants, calcium hydroxide is used to neutralize acidic wastewater, raising the pH to a more environmentally acceptable level. It also aids in the precipitation of heavy metals and other pollutants, facilitating their removal from the water.
-
Agriculture: Calcium hydroxide is applied to acidic soils to increase their pH and improve nutrient availability for plants. This practice is crucial for optimal crop growth and yield.
-
Food Industry: In food processing, calcium hydroxide is used as a food additive, a firming agent for vegetables and fruits, and in the production of certain food products. It's a crucial component in traditional food preparations like nixtamalization of corn (making tortillas).
-
Chemical Industry: Calcium hydroxide serves as a starting material for various chemical reactions, including the production of other calcium compounds and the synthesis of specific chemicals.
-
Paper Production: In the paper industry, it's utilized in the pulp and paper manufacturing process to control the pH of the pulp slurry.
-
Sugar Refining: Calcium hydroxide is involved in the purification of sugar, helping to remove impurities.
Safety Considerations for Handling Calcium Hydroxide
While calcium hydroxide is relatively safe when handled correctly, certain precautions should be taken to prevent potential hazards:
-
Eye Protection: Always wear appropriate eye protection, such as safety goggles, to avoid irritation or damage to the eyes. Direct contact with calcium hydroxide can cause significant eye irritation.
-
Respiratory Protection: Inhaling calcium hydroxide dust can cause respiratory irritation. A dust mask or respirator should be used, especially in environments with high dust concentrations.
-
Skin Protection: Direct contact with skin can lead to irritation and burns. Protective gloves and clothing should be worn when handling calcium hydroxide.
-
Storage: Store calcium hydroxide in a dry, well-ventilated area, away from incompatible materials. Keep containers tightly sealed to prevent moisture absorption.
-
Disposal: Follow local regulations for the safe disposal of calcium hydroxide waste. Proper disposal methods are crucial to avoid environmental contamination.
Scientific Explanation: The Chemistry Behind Calcium Hydroxide's Properties
The properties and uses of calcium hydroxide stem directly from its chemical structure and reactivity. The strong ionic bond between the calcium cation (Ca²⁺) and the hydroxide anions (OH⁻) is responsible for its unique characteristics.
-
Alkalinity: The hydroxide ion (OH⁻) is a strong base. When calcium hydroxide dissolves in water, it dissociates into calcium ions and hydroxide ions, increasing the concentration of OH⁻ in the solution and making it alkaline. This alkaline nature is responsible for its use in neutralizing acids.
-
Reactivity with Acids: Calcium hydroxide readily reacts with acids in a neutralization reaction, forming a salt and water. This property is utilized in various applications, such as wastewater treatment and soil pH adjustment. A typical neutralization reaction is:
Ca(OH)₂ + 2HCl → CaCl₂ + 2H₂O
-
Solubility: The relatively low solubility of calcium hydroxide in water is due to the strong lattice energy of its crystalline structure. The strong electrostatic attraction between the calcium and hydroxide ions makes it difficult for the compound to dissolve completely.
-
Thermal Decomposition: At high temperatures, calcium hydroxide undergoes thermal decomposition, losing water and forming calcium oxide (CaO), also known as quicklime.
Ca(OH)₂ → CaO + H₂O
Frequently Asked Questions (FAQ)
Q1: What is the difference between quicklime and slaked lime?
A1: Quicklime (CaO) is calcium oxide, an anhydrous compound. Slaked lime (Ca(OH)₂) is calcium hydroxide, formed by the reaction of quicklime with water. Slaked lime is the hydrated form of quicklime.
Q2: Can I use calcium hydroxide to adjust the pH of my swimming pool?
A2: While calcium hydroxide can increase the pH of water, it's generally not recommended for swimming pools. Other chemicals specifically designed for pool pH adjustment are safer and more effective. Improper use can lead to clouding, scaling, and other issues.
Q3: Is calcium hydroxide harmful to the environment?
A3: In large quantities or improperly disposed of, calcium hydroxide can have negative environmental impacts, such as altering soil pH or contaminating water sources. However, when used responsibly and in accordance with regulations, its environmental impact is minimal.
Q4: Where can I buy calcium hydroxide?
A4: Calcium hydroxide is widely available from chemical suppliers, building supply stores, and online retailers. Always ensure that you are purchasing from a reputable source.
Q5: What are the potential health hazards associated with calcium hydroxide?
A5: Contact with eyes or skin can cause irritation or burns. Inhalation of dust can cause respiratory irritation. Ingestion can cause serious internal damage. Always wear appropriate personal protective equipment (PPE) when handling calcium hydroxide.
Conclusion
Calcium hydroxide (Ca(OH)₂) is a versatile and widely used compound with a molar mass of approximately 74.10 g/mol. Understanding its molar mass is fundamental to various scientific and industrial applications. Its properties, uses, and safety precautions are crucial for anyone working with this compound. From building construction to wastewater treatment and food processing, calcium hydroxide plays a significant role in many aspects of modern life. Always prioritize safe handling practices and adhere to relevant safety regulations when working with calcium hydroxide. This guide provides a foundation for a deeper understanding of this important chemical compound, emphasizing the importance of responsible use and safe handling procedures.
Latest Posts
Latest Posts
-
How Long Does Photosynthesis Take
Sep 12, 2025
-
Is Co Ionic Or Covalent
Sep 12, 2025
-
Whats 20 Kg In Pounds
Sep 12, 2025
-
Is Shampoo Base Or Acid
Sep 12, 2025
-
To Cause Cancer Proto Oncogenes Require
Sep 12, 2025
Related Post
Thank you for visiting our website which covers about Ca Oh 2 Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.