0.5 Ppm To Mg Kg
salachar
Sep 14, 2025 · 7 min read
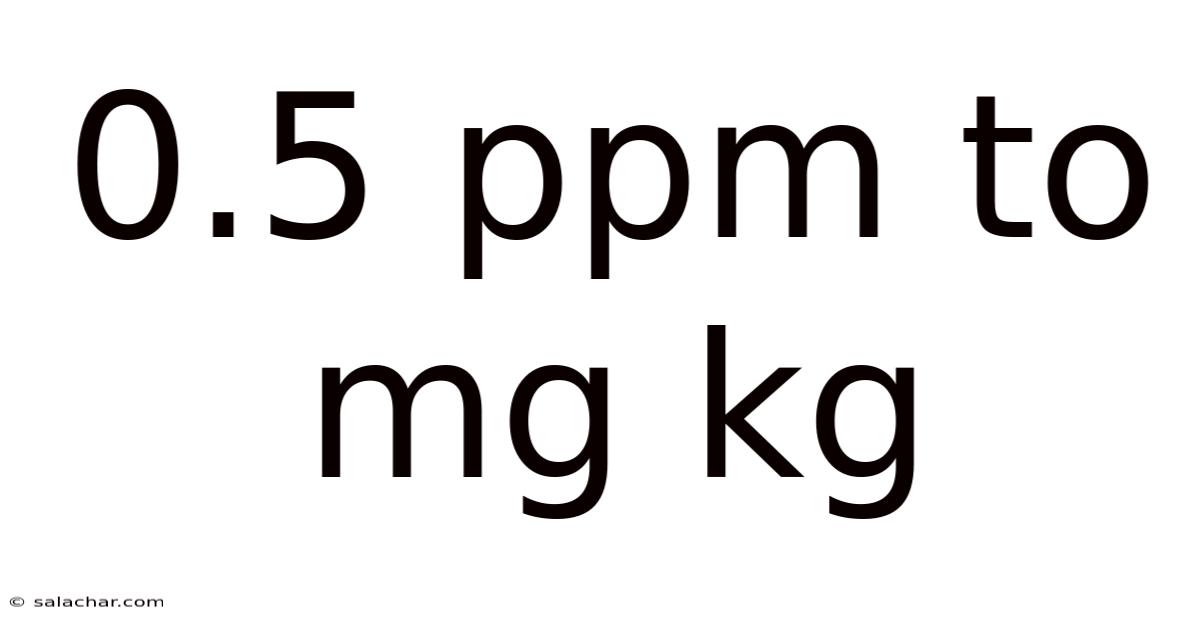
Table of Contents
Understanding the Conversion: 0.5 ppm to mg/kg
Converting units is a fundamental skill in many scientific and engineering fields. This article will delve into the conversion of parts per million (ppm) to milligrams per kilogram (mg/kg), focusing specifically on understanding the relationship between these units and applying the conversion to a real-world example of 0.5 ppm. We will explore the underlying principles, provide a step-by-step guide, and address frequently asked questions to ensure a thorough comprehension of this important concept. Understanding this conversion is crucial in various applications, from environmental monitoring (analyzing pollutant concentrations in soil or water) to food safety (determining the presence of contaminants).
Introduction: ppm and mg/kg – A Deep Dive
Parts per million (ppm) and milligrams per kilogram (mg/kg) are both units of concentration, expressing the amount of a solute (the substance being dissolved) within a solvent (the substance doing the dissolving). They are commonly used to express very low concentrations, making them particularly relevant for trace elements or contaminants. While seemingly different, they represent the same concept – the ratio of solute to solvent – just using different scales.
-
Parts per million (ppm): This unit expresses the number of units of a substance per million units of a mixture. It's a dimensionless ratio, often used for expressing small concentrations in various contexts, including water quality, air pollution, and materials science. For example, 0.5 ppm of a contaminant in water means that there are 0.5 parts of the contaminant for every 1 million parts of water.
-
Milligrams per kilogram (mg/kg): This unit expresses the mass of a solute in milligrams (mg) per kilogram (kg) of the solvent or mixture. It's a unit of concentration representing the mass ratio of the substance to the total mass. It's frequently used in environmental chemistry, toxicology, and food science.
The key to understanding the conversion lies in recognizing that ppm and mg/kg are essentially equivalent when dealing with solutions where the density is approximately 1 kg/L (like water). This equivalence is due to the consistent relationship between milligrams, kilograms, and the numerical base of ppm (1 million).
The Conversion: 0.5 ppm to mg/kg - A Step-by-Step Guide
The conversion from ppm to mg/kg is remarkably straightforward when the density of the solution is approximately 1 kg/L. Under this condition, 1 ppm is equivalent to 1 mg/kg. Therefore, converting 0.5 ppm to mg/kg simply involves a direct substitution:
0.5 ppm = 0.5 mg/kg
This means that if you have a solution with a concentration of 0.5 ppm of a particular substance, you also have a concentration of 0.5 mg/kg of that same substance. This direct relationship simplifies the conversion significantly.
However, it's crucial to note that this direct conversion is only valid when the density of the solution is approximately 1 kg/L. If the density differs significantly from 1 kg/L (for example, a dense solution like concentrated acid or a less dense solution like certain organic solvents), a density correction factor must be applied to the conversion calculation. The formula for a more general case is:
mg/kg = ppm * (density of the solution in kg/L)
For our example of 0.5 ppm, if we were dealing with a solution with a density other than 1 kg/L, we'd need to use this formula. For example, if the density was 1.2 kg/L, the calculation would be:
mg/kg = 0.5 ppm * 1.2 kg/L = 0.6 mg/kg
This demonstrates that the density of the solution plays a vital role in the conversion, especially when working with substances that have significantly different densities than water.
Practical Applications and Examples
The conversion between ppm and mg/kg is fundamental in numerous fields. Here are some examples:
-
Environmental Monitoring: Determining the concentration of heavy metals (e.g., lead, mercury) in soil or water samples. A measurement of 0.5 ppm lead in soil would translate to 0.5 mg/kg, indicating a relatively low level of contamination (assuming a soil density close to 1 kg/L).
-
Food Safety: Assessing the presence of pesticide residues in food products. A regulatory limit of 0.5 ppm of a particular pesticide would be equivalent to 0.5 mg/kg, providing a clear guideline for food safety standards.
-
Pharmaceutical Industry: Controlling the concentration of active ingredients in medications. Maintaining accurate concentration levels is critical for efficacy and safety, and ppm/mg/kg conversion is often necessary for dosage calculations and quality control.
-
Industrial Processes: Monitoring the concentration of trace elements in materials. For example, ensuring the precise composition of alloys or semiconductors often requires accurate conversion between different concentration units.
The Scientific Basis: Understanding Units and Dimensional Analysis
The equivalence between ppm and mg/kg, when density is approximately 1 kg/L, stems from the definition of these units and the fundamental relationships between mass and volume.
-
ppm is a dimensionless ratio, expressing a proportion.
-
mg/kg is a ratio of mass (mg) to mass (kg).
Since 1 kg = 1,000,000 mg (1 million milligrams), the ratio of milligrams to kilograms can be expressed as a part per million relationship. This fundamental relationship facilitates the direct conversion when the density is 1 kg/L.
Dimensional analysis further clarifies this:
-
We start with 0.5 ppm. This can be expressed as: 0.5 (mg/L) / (1,000,000 mg/L) = 0.5 * 10<sup>-6</sup>
-
If the density is 1 kg/L, then the liters cancel out, leaving us with: 0.5 * 10<sup>-6</sup> kg = 0.5 * 10<sup>-3</sup> g = 0.5 mg per kg
This illustrates the mathematical basis for the simple conversion when the density is approximately 1 kg/L.
Frequently Asked Questions (FAQs)
Q1: What if the density of the solution is not 1 kg/L?
A: If the density is different from 1 kg/L, you must use the formula: mg/kg = ppm * (density of the solution in kg/L). The density is crucial for accurate conversion.
Q2: Are there other units that express similar concentrations?
A: Yes, other units like parts per billion (ppb) and parts per trillion (ppt) express even lower concentrations. These are also directly convertible to mg/kg, mg/L (milligrams per liter), or µg/L (micrograms per liter) with appropriate adjustments based on the solution's density.
Q3: How precise does the density measurement need to be for accurate conversion?
A: The required precision of the density measurement depends on the accuracy needed for the final mg/kg concentration. For high-precision work, a more precise density determination is necessary.
Q4: Can this conversion be applied to gases?
A: The conversion from ppm to mg/kg can be applied to gases, but it requires considering the gas density under specific conditions (temperature and pressure) and using the ideal gas law or other relevant equations of state to calculate the mass concentration. The direct 1:1 conversion is not applicable to gases without these adjustments.
Conclusion: Mastering Unit Conversions for Accurate Results
Mastering the conversion between ppm and mg/kg is essential for accurate interpretation and reporting of concentration data across numerous scientific and engineering disciplines. While the direct conversion (0.5 ppm = 0.5 mg/kg) is convenient when the density is near 1 kg/L, it's crucial to remember the importance of considering the solution's density for accurate results in other cases. By understanding the underlying principles and applying the appropriate formula, you can confidently navigate unit conversions and ensure the accurate interpretation of concentration data. This knowledge is not only vital for academic understanding but also for making informed decisions in various practical applications, from environmental protection to ensuring product safety and quality control. Remember to always double-check your calculations and consider the context of the problem for accurate interpretation and reporting.
Latest Posts
Latest Posts
-
Midsummer Nights Dream Puck Monologue
Sep 14, 2025
-
Differentiate Between Exocytosis And Endocytosis
Sep 14, 2025
-
Square Root Of 289 Simplified
Sep 14, 2025
-
Toys That Start With Ak
Sep 14, 2025
-
Exergonic Reaction Vs Endergonic Reaction
Sep 14, 2025
Related Post
Thank you for visiting our website which covers about 0.5 Ppm To Mg Kg . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.