Balance Na H2o Naoh H2
salachar
Sep 02, 2025 · 6 min read
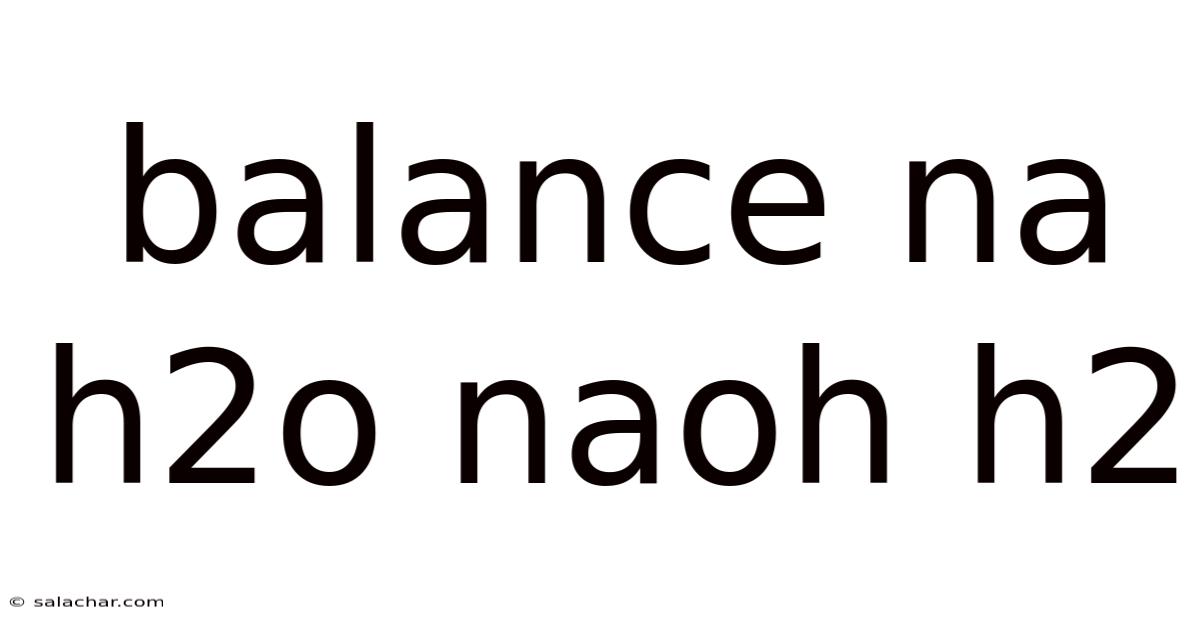
Table of Contents
Understanding the Balancing Act: Na + H₂O + NaOH + H₂
This article explores the chemical reaction between sodium (Na) and water (H₂O), resulting in the formation of sodium hydroxide (NaOH) and hydrogen gas (H₂). We'll delve into the balanced chemical equation, the underlying mechanisms, safety precautions, and practical applications of this highly reactive process. Understanding this reaction is crucial for anyone studying chemistry, particularly introductory chemistry and its practical applications.
Introduction: A Reactive Encounter
The reaction between sodium metal and water is a classic example of a highly exothermic redox reaction. Sodium, an alkali metal, is highly reactive due to its low ionization energy and strong tendency to lose an electron. Water, while seemingly benign, acts as both an oxidant and a source of protons (H⁺). When these two substances meet, a vigorous reaction ensues, releasing a significant amount of energy in the form of heat and often igniting the hydrogen gas produced. This reaction is represented by the unbalanced equation: Na + H₂O → NaOH + H₂. Our primary focus will be on understanding how to balance this equation and the intricate details behind it.
Balancing the Chemical Equation: A Step-by-Step Guide
Balancing chemical equations is crucial in chemistry as it ensures the conservation of mass. The law of conservation of mass dictates that the number of atoms of each element must be the same on both sides of the equation. Let's balance the equation for the reaction between sodium and water:
Unbalanced Equation: Na + H₂O → NaOH + H₂
Step 1: Identify the Elements
We have sodium (Na), hydrogen (H), and oxygen (O) involved in this reaction.
Step 2: Count Atoms on Each Side
- Reactants (left side): 1 Na, 2 H, 1 O
- Products (right side): 1 Na, 3 H, 1 O
Step 3: Balance the Equation
Notice that hydrogen is unbalanced. There are two hydrogen atoms on the reactant side and three on the product side. To balance the hydrogen atoms, we can start by adding a coefficient of 2 in front of H₂O on the reactant side. This gives us:
Na + 2H₂O → NaOH + H₂
Now let's recount the atoms:
- Reactants: 1 Na, 4 H, 2 O
- Products: 1 Na, 3 H, 1 O
The oxygen is still unbalanced. To balance the oxygen, we add a coefficient of 2 in front of NaOH on the product side:
Na + 2H₂O → 2NaOH + H₂
Recounting again:
- Reactants: 1 Na, 4 H, 2 O
- Products: 2 Na, 4 H, 2 O
The sodium is now unbalanced. To resolve this, we add a coefficient of 2 in front of Na on the reactant side:
Balanced Equation: 2Na + 2H₂O → 2NaOH + H₂
Now the equation is balanced! We have 2 Na atoms, 4 H atoms, and 2 O atoms on both the reactant and product sides.
The Scientific Explanation: Redox Reaction and Energetics
This reaction is a classic example of a redox reaction, which involves both reduction and oxidation processes. Sodium undergoes oxidation, losing one electron to become a sodium ion (Na⁺). The water molecules act as oxidizing agents, accepting the electrons released by sodium. Simultaneously, water is reduced, forming hydrogen gas. The hydrogen atoms gain electrons to form H₂.
The reaction is highly exothermic due to the significant energy difference between the reactants and products. The ionic bonds formed in sodium hydroxide (NaOH) are much stronger than the metallic bond in sodium and the covalent bonds in water. This energy difference is released as heat, often causing the hydrogen gas to ignite spontaneously. The intense heat generated during this reaction is due to the strong electrostatic attraction between the positively charged sodium ions (Na⁺) and the negatively charged hydroxide ions (OH⁻) in the newly formed sodium hydroxide. The enthalpy change (ΔH) for this reaction is highly negative, indicating a large release of heat.
Safety Precautions: Handling Sodium with Care
Sodium metal is extremely reactive with water, posing significant safety hazards. Never attempt to perform this reaction without proper training and safety equipment. Here are some key precautions:
- Wear appropriate personal protective equipment (PPE): This includes safety goggles, gloves, and a lab coat.
- Perform the reaction in a well-ventilated area: The hydrogen gas produced is flammable and explosive.
- Use small quantities of sodium: A small piece of sodium (a few milligrams) is sufficient to demonstrate the reaction. Larger quantities can lead to a more violent reaction and potential hazards.
- Use a suitable container: A large beaker or a heat-resistant container is recommended.
- Never touch sodium with bare hands: It can cause severe burns.
- Have a fire extinguisher readily available: In case of an uncontrolled reaction or fire.
- Proper disposal of waste: Sodium hydroxide solution is a strong base and requires careful disposal following established laboratory protocols.
Practical Applications: Beyond the Laboratory
While the reaction itself might seem like a simple demonstration, its principles find applications in various fields:
- Industrial production of sodium hydroxide: Although industrial production of NaOH typically uses electrolysis of brine (sodium chloride solution), the fundamental principle of the reaction of sodium with water is relevant to understanding its chemical basis.
- Hydrogen production: Although not a practical method for large-scale hydrogen production due to the cost and safety concerns of handling sodium metal, this reaction highlights the possibility of producing hydrogen gas from a readily available metal and water.
- Educational purposes: The reaction serves as a fascinating and visually impressive demonstration in chemistry classrooms to illustrate concepts of redox reactions, exothermic reactions, and the reactivity of alkali metals.
Frequently Asked Questions (FAQ)
Q1: Why is the reaction so vigorous?
A1: The reaction is vigorous due to the high reactivity of sodium, its strong tendency to lose electrons, and the exothermic nature of the reaction. The energy released is sufficient to ignite the hydrogen gas produced.
Q2: Can other alkali metals react similarly with water?
A2: Yes, other alkali metals like lithium (Li), potassium (K), rubidium (Rb), and cesium (Cs) also react vigorously with water, but the intensity of the reaction increases as you go down the group in the periodic table. Cesium reacts explosively with water.
Q3: What are the products of this reaction?
A3: The products of the reaction are sodium hydroxide (NaOH), a strong base, and hydrogen gas (H₂), a flammable gas.
Q4: What is the role of water in this reaction?
A4: Water acts as both an oxidizing agent (accepting electrons from sodium) and a source of protons (H⁺) which are reduced to form hydrogen gas.
Q5: Is this reaction safe to perform at home?
A5: No, this reaction is not safe to perform at home without proper safety equipment, training, and a controlled environment. The risks of burns, fire, and explosion are significant.
Conclusion: A Powerful Demonstration of Chemical Principles
The reaction between sodium and water, represented by the balanced equation 2Na + 2H₂O → 2NaOH + H₂, offers a captivating demonstration of fundamental chemical principles. It vividly illustrates the concepts of redox reactions, exothermic reactions, and the reactivity of alkali metals. However, it's crucial to remember the safety implications involved and to always handle sodium metal with extreme caution in a controlled laboratory setting with proper safety equipment. Understanding this reaction deepens our comprehension of chemical reactivity and its practical implications, spanning from industrial processes to educational demonstrations. The careful study of this seemingly simple reaction reveals a complex interplay of chemical forces, emphasizing the importance of balanced equations and safety protocols in the world of chemistry.
Latest Posts
Latest Posts
-
Difference Between Population And Species
Sep 02, 2025
-
Whats 5 Percent Of 500
Sep 02, 2025
-
Blank Of The Rising Sun
Sep 02, 2025
-
What Is A Base Price
Sep 02, 2025
-
Surface Area Of A Pipe
Sep 02, 2025
Related Post
Thank you for visiting our website which covers about Balance Na H2o Naoh H2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.